OUR PIPELINE

Using our patented heart therapeutic, ISX9-CPC creates new cardiac muscle and replaces dead scar tissue and restore cardiac function. While our published efficacy applies to the larger MI/heart failure population, our initial clinical focus will be to prove our therapy in a smaller patient population in duchenne cardiomyopathy which is also the ultimate source of death for DMD patients. Given our completed FDA PRE-IND meeting & ongoing GMP manufacturing program that started in 2019, we believe we will have the first IND in the United States with ISX9-CPC. In addition, the FDA has granted us orphan designation in 2022.
We are advancing a pipeline of two single-course stem cell therapeutics intended to safely and durably create new heart muscle and new skeletal muscle in DMD patients. We aim to later expand clinically beyond duchenne cardiomyopathy to the larger core MI/heart failure market with ISX9-CPC and will also expand beyond DMD patients for GIVI-MPC to other rare muscle diseases.
ENCOURAGING PRE-CLINICAL RESULTS USING HUMAN CELLS TO CREATE NEW HUMAN MUSCLE IN MICE AND PIGS
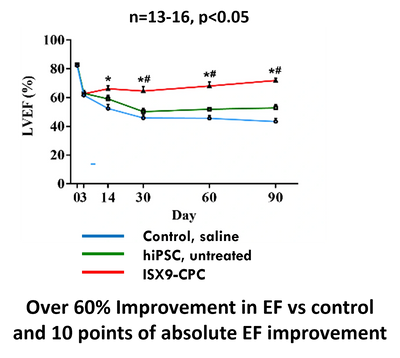
With ISX9-CPC, in an in vivo proof-of-concept peer reviewed study of a single treatment of our patented ISX9-CPC's reduced dead scar tissue by 70% and restored heart function by over 60% EF improvement vs. control 90 days after heart attack. With GIVI-MPC, we created significant new human skeletal muscle along with 100% full length human dystrophin in mdx mice and in dystrophic pigs.
ISX9-CPC RESULTED IN REDUCED INFARCT&Improved heart functiON
Proven Heart Regeneration
- 66% Ejection fraction increase vs control
- 9 points higher absolute EF 90 days after heart attack (which compares to 2% average for previous human clinical stem cell trials)
- Scar tissue reduction from 27% to 7%
800% more cardiomyocytes 3 months out vs control
30% proven engraftment 3 days out

GIVI-MPC RESULTED IN New skeletal muscle with 100% length dystrophin
Copyright © 2014 IPS HEART - All Rights Reserved.
.png/:/rs=h:75,cg:true,m/qt=q:95)